- How many electrons can fit into the third shell?
- 18 (2 s, 6 p, and 10 d)
- What are the possible values for the azimuthal quantum number, ℓ, when n = 2?
- 0 ≤ ℓ ≤ n-1 ⇒ ℓ = 0 or ℓ = 1 (s and p)
- What are the possible values of the magnetic quantum number, mℓ, when ℓ = 1?
- -ℓ ≤ mℓ ≤ ℓ ⇒ mℓ = -1, 0, 1
- What are n and ℓ for 2p orbitals?
- n = 2, ℓ = 1
- What are n and ℓ for 3s orbitals?
- n = 3, ℓ = 0
- Answer
- Answer
- Answer
- Answer
- Answer
- the outmost electron in Ca.
- n = 4, ℓ = 0 (s), mℓ = 0, ms = ½, -½
- the electron in the first excited state of Ca. The first excited state occurs when the outermost electron is promoted to the next higher energy level.
- n = 4, ℓ = 1 (p); mℓ = -1, 0, 1; ms = ½, -½
- a d-subshell electron in Ag.
- n = 4, ℓ = 2 (d); mℓ = -2, -1, 0, 1, 2; ms = ½, -½
- Answer
- Answer
- Answer
- +3, [Kr]4d5
- Ru3+
- +2, [Xe]
- Ba2+
- +1, [Ar]3d10
- Cu1+
- -1, [Kr]
- Br1-
- Answer
- Answer
- Answer
- Answer
- Ca
- [Ar]4s2
- S
- [Ne]3s23p4
- Sr2+
- [Kr]
- Fe3+
- [Ar]3d5
- Ag
- [Kr]5s14d10
- Answer
- Answer
- Answer
- Answer
- Answer
Periodic Trends (Ch. 7)
- Answer
- The inner electrons and the two 3s electrons would shield all but one of the protons for Al, giving it an effective nuclear charge of +1. Assuming the p electrons don't shield each other, each element would gain an effective nuclear charge of +1 above the last element. So, Si would have +2, P would have +3, S would have +4, and Cl would have +5.
If you assume the 3s electrons don't shield, the Al atom would have an effective nuclear charge of +3, and so Si would have +4, P would have +5, S would have +6, and Cl would have +7.
- Answer
- Cl would have the smaller atom. As stated in the last problem the effective nuclear charge increases as we go across the table. The larger the effective nuclear charge, the stronger the pull on the electron toward the nucleus and the smaller the atomic size.
- Answer
- (a) Going down the table generally increases the number of protons and electrons so much that the atoms become bigger as we go down the chart. (b) Adding an electron generally makes the ion larger. (c) Taking away an electron generally makes the ion smaller.
- Answer
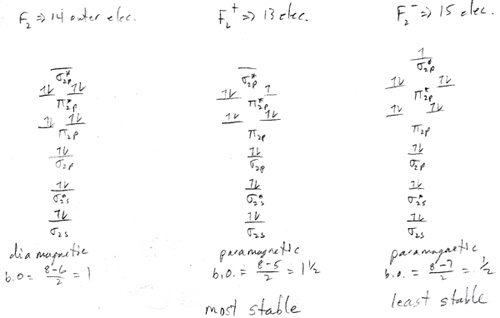
- (a) Be, it has a full subshell while B can give one up to have a full subshell. (b) As, it has a half full subshell while Se can give one up to have a half full subshell. (c) Br, it tries to gain an electron while Ca is ready to give up electrons.
- Answer
-
- Answer
- Mg has a full subshell requiring energy to put another electron into the atom. Na can accept an electron to have a full subshell, even though it prefers to give up an electron to become like a noble gas.
- Answer
- Xe has a full subshell requiring energy to put another electron into the atom. Iodine can accept an electron to have a full subshell.
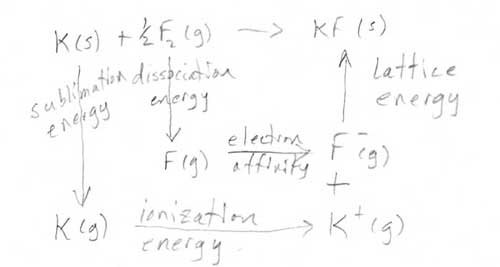
Basic Bonding Concepts (Ch. 8)
(a) Li, Be, Na, Mg and (b) As, Se, Sb, Te.
- Answer
- (a) Be, (b) Se
- Answer
-
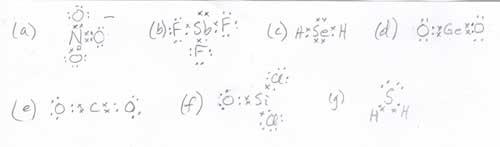
- Answer
-
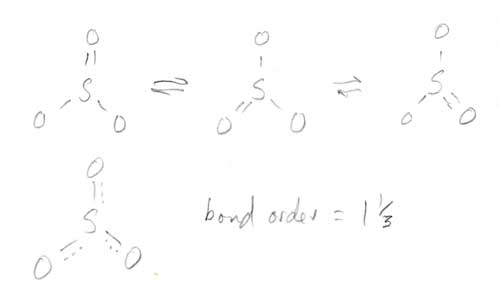
Bonding Theories (Ch. 9)
- Answer
-
AXE Hybrid Orbital E-Domain Shape Molecular Shape SeOBr2 AX3E sp3 Tetrahedral Trigonal Pyramidal BrI4- AX4E2 sp3d2 Octahedral Square Pyramidal BeCl2 AX2 sp Linear Linear PH4+ AX4 sp3 Tetrahedral Tetrahedral SeI4 AX4E sp3d Trigonal Bipyramidal Seesaw XeCl2 AX2E3 sp3 Trigonal Bipyramidal Linear IBr3 AX3E2 sp3d Trigonal Bipyramidal T-Shaped AlH3 AX3E sp3 Tetrahedral Trigonal Pyramidal
- Answer
-