- Answer
- The electrons have shifted to one side of the molecule.
- Answer
-
(a) HF
(b) CH3Cl
- Answer
- (a) I2 (b) C10H22
- Answer
- Propanol is larger and will have more London Dispersion Forces giving it stonger intermolecular forces and requiring more energy to separate the molecules. Propanol also has more mass and that also requires more energy to move them around and separate them. Both of these will contribute to a higher boiling point for propanol.
- Answer
- The ethylene glycol can form hydrogen bonds on both ends of the molecule resulting in much stronger intermolecular forces and a higher boiling point.
- Answer
- (a) I2, it is the largest nonpolar molecule. (b) H2S, S is more electronegative and will make the molecule more polar.
- Answer
- (a) Nonpolar - London Dispersion Forces. (b) Polar - Dipole-dipole attractions (and dispersion forces). (c) Very polar - Hydrogen bonds between the molecules. Hydrogen bonds form when hydrogen is covalently bonded to N, O, or F. (d) No molecules, so there are no intermolecular forces - Ionic bonds.
- NH3 (-33.35 °C) and PH3 (-87.7 °C)
- According to polarity the more polar ammonia, NH3, should be harder to boil (which it is). According to mass ammonia, NH3, should be easier to boil (but it isn't). In this case the effect of polarity is greater than the effect of mass.
- PH3 (-87.7 °C) and AsH3 (-55 °C)
- PH3 is more polar (P is closer to F) and AsH3 has more mass. According to polarity AsH3 should boil at a lower temperature, but according to mass PH3 should boil at a lower temperature. In this case the mass effect is greater than the polarity effect.
- HCl (-85 °C) and HBr (-66 °C)
- HCl is more polar, but the increased mass of HBr gives it a higher boiling point.
- Br2 (58.78 °C) and I2 (184.35 °C)
- Both are nonpolar, but I2 has more mass and it is more polarizable (stronger intermolecular forces), so it will have the higher boiling point.
- HF (19.54 °C) and Ne (-245.92 °C)
- Both have about the same molecular weight, but HF is very polar, so HF has the higher boiling point.
- Answer
- Answer
- Answer
- Answer
- Answer
- Answer
- (a) I2, it is the largest nonpolar molecule. (b) H2S, S is more electronegative and will make the molecule more polar.
- Answer
-
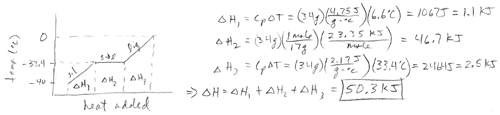
- Answer
-
| Crystal | Melting Point (°C) | Boiling Point (°C) | Electrical Conductivity Solid Liquid | |
|---|---|---|---|---|
| A | -83.1 | 19.54 | No | No |
| B | -259.14 | -252.5 | No | No |
| C | 1535 | 3000 | Yes | Yes |
| D | 686 | 1330 | No | Yes |
| E | -56.6 | -78.5 | No | No |
| F | -182.48 | -164.8 | No | No |
| G | 3550 | 4827 | No | No |
- Answer
-
A ⇒ good separation between melting and boiling points, relatively high m.p. for molecular, no conductivity - polar-molecular. (HF - polar molecule)
B ⇒ close and low melting and boiling points, no condutivity - nonpolar-molecular. (H2 - nonpolar molecule)
C ⇒ high mp and bp, good conductor - metallic. (Fe - metal)
D ⇒ high melting and boiling points, conducts as a liquid - ionic. (KI - ionic compound)
E ⇒ low and close together mp and bp, nonconductor - nonpolar-molecular. (CO2 - nonpolar molecule)
F ⇒ low and close together mp and bp, nonconductor - nonpolar-molecular. (CH4 - nonpolar molecule)
G ⇒ very high mp and bp, nonconductor - covalent (network). (Diamond - covalent network)
- Answer
-
HF - polar molecule, good separation between melting and boiling points, relatively high m.p. for molecular, no conductivity - polar-molecular.
H2 - nonpolar molecule, close and low melting and boiling points, no condutivity - nonpolar-molecular.
Fe - metal, high or low mp and bp, good conductor - metallic.
KI - ionic compound, high melting and boiling points, conducts as a liquid - ionic.
CO2 - nonpolar molecule, low and close together mp and bp, nonconductor - nonpolar-molecular.
CH4 - nonpolar molecule, low and close together mp and bp, nonconductor - nonpolar-molecular.
Diamond - covalent network, very high mp and bp, nonconductor - covalent (network)
Crystal Melting Point (°C) Boiling Point (°C) Electrical Conductivity
Solid LiquidHF -83.1 19.54 No No H2 -259.14 -252.5 No No Fe 1535 3000 Yes Yes KI 686 1330 No Yes CO2 -56.6 -78.5 No No CH4 -182.48 -164.8 No No Diamond 3550 4827 No No
- Answer
- (58.7g/mole)(1 mole/6.02x1023 atoms)(4 atoms/unit cell)(1 unit cell/[3.525x10-8]3 cm3) = 8.9 g/cm3
- Answer
- (6.02 x 1023 atoms/mole)(1 mole/107.9 g)(10.5 g/cm3)([4.09 x 10-8 cm]3/unit cell) = 4 atoms/unit cell ⇒ fcc.